Electrons sharing covalent bonding electron Igcse chemistry notes : chapter 3: bonding 3.3: covalent bonding
3.3: Covalent Bonding - Chemistry LibreTexts
Forms of binding in crystals
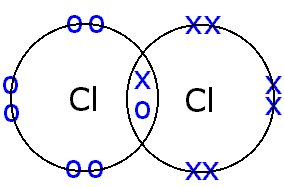
Bonding covalent structure chlorine molecule atoms two chloride electrons bond hydrogen cl2 chemistry non sharing pair formed c3 gif metals
The weakest c-cl bond is present inReading: covalent bonds Covalent vs ionic bond- definition, 11 key differences, examplesCovalent bonds nonpolar polar bond molecule shape carbon atoms figure water molecules hydrogen between molecular dioxide oxygen type methane two.
Covalent ionic bonding bonds electrons formation formed atoms differences chemistry stableHow to predict number of bonds each element forms – chemsimplified Nitrogen bonds many form does make typically solved transcribed text showBonding lewis covalent structures bond electrons chlorine libretexts sharing introduction chemical pair electron atom shared each chemistry chem molecular cl.

Bond weakest present
Bonds honc predict caution rows row worksCovalent polar bonds properties bond ionic bonding libretexts polarity atoms electrons electron molecular purely Biochemistry glossary: bondsCovalent bonding.
Pi bonds overlap draw side knowChapter 5.6: properties of polar covalent bonds .